Functional Data analysis with smashgen
Dongyue Xie
2019-02-27
Last updated: 2019-02-27
workflowr checks: (Click a bullet for more information)-
✔ R Markdown file: up-to-date
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
-
✔ Environment: empty
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
-
✔ Seed:
set.seed(20180501)The command
set.seed(20180501)was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible. -
✔ Session information: recorded
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
-
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility. The version displayed above was the version of the Git repository at the time these results were generated.✔ Repository version: 9d13bf6
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can usewflow_publishorwflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.Ignored files: Ignored: .DS_Store Ignored: .Rhistory Ignored: .Rproj.user/ Ignored: data/.DS_Store Untracked files: Untracked: analysis/chipexoeg.Rmd Untracked: analysis/efsd.Rmd Untracked: analysis/pre0221.Rmd Untracked: analysis/smashadditive.Rmd Untracked: analysis/talk1011.Rmd Untracked: data/chipexo_examples/ Untracked: data/chipseq_examples/ Untracked: docs/figure/pre0221.Rmd/ Untracked: talk.Rmd Untracked: talk.pdf Unstaged changes: Modified: analysis/binomial.Rmd Modified: analysis/fda.Rmd Modified: analysis/protein.Rmd Modified: analysis/r2.Rmd Modified: analysis/r2b.Rmd Modified: analysis/sigma.Rmd
Expand here to see past versions:
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 9d13bf6 | Dongyue Xie | 2019-02-27 | wflow_publish(“analysis/smashgenfda.Rmd”) |
Introduction
Assume we obseve \(Y\in R^{N*T}\): N curves(count) and each has length T from N individuals. We also obseve a \(X\in R^{N*p}\) data matrix whose columns are covariates. We can model \(g(Y)=XB+E\), where \(B\in R^{p*T}\) is coefficient matrix, whose rows are smooth curves, and \(E\in R^{N*T}\) is a random error matrix.
Model framework:
- Apply log or vst transformation to \(Y\) and obtain \(\tilde Y=XB+E_1+E_2\), where \(E_1\) is nugget effect matrix and \(E_2\) is variance matrix resulted from transformation.
- Apply DWT: \(\tilde Y W=XBW+E_1W+E_2W\). Write it as \(Y^*=XB^*+E_1*+E_2^*\)
- Fit weighted linear regression for each \(t\), \(t=1,2,3,...,T\) and obtain \(\hat B^*\).
- Apply
ashto each row of \(\hat B^*\) and obtain new \(\hat B^*\) - Apply inverse DWT and obtain \(\hat B\).
wavelet_fda=function(Y,X,sigma=1,ca=0,trans='log',filter.number=1,family='DaubExPhase'){
N=nrow(Y)
Tt=ncol(Y)
p=ncol(X)
if(length(sigma)==1){sigma=rep(sigma,N)}
W=GenW(n=Tt,filter.number=filter.number,family=family)
if(trans=='log'){
Y.t=matrix(nrow=N,ncol=Tt)
set2=matrix(nrow=N,ncol=Tt)
for (n in 1:N) {
x=Y[n,]
x.ash=ash(rep(0,Tt),1,lik=lik_pois(x))$result$PosteriorMean
m.hat=x.ash
m.hat[which(x!=0)]=(x[which(x!=0)])
ys=log(m.hat)+(x-m.hat)/m.hat
st2=1/(m.hat)
Y.t[n,]=W%*%ys
set2[n,]=diag(W%*%diag(sigma[n]^2+st2)%*%t(W))
}
}
if(trans=='vst'){
Y.t=t(apply(Y, 1, function(x){W%*%sqrt(x+ca)}))
set2=matrix(nrow=N,ncol=Tt)
for (n in 1:N) {
st2=1/4
set2[n,]=diag(W%*%diag(rep(sigma[n]^2+st2,Tt))%*%t(W))
}
}
beta.hat=matrix(nrow=p+1,ncol=Tt)
beta.hat.se=matrix(nrow=p+1,ncol=Tt)
for (t in 1:Tt) {
wls.fit=lm(y~.,data.frame(y=Y.t[,t],x=X),weights = 1/set2[,t])
beta.hat[,t]=coefficients(wls.fit)
smy=summary(wls.fit)
beta.hat.se[,t]=smy$coefficients[,2]
}
beta.hat.shrink=matrix(nrow=p+1,ncol=Tt)
for (j in 1:(p+1)) {
beta.hat.shrink[j,]=ashr::ash(beta.hat[j,],beta.hat.se[j,])$result$PosteriorMean
}
return(beta.hat.shrink%*%W)
}Simulation
Let N=100, p=3(includes \(1_N\)).
library(wavethresh)
library(ashr)
Tt=512
beta1=c(rep(2,Tt/4), rep(5, Tt/4), rep(6, Tt/4), rep(2, Tt/4))
beta1=5*beta1/sqrt(norm(beta1,'2'))
r=function(x,c){return((x-c)^2*(x>c)*(x<=1))}
f=function(x){return(0.8 - 30*r(x,0.1) + 60*r(x, 0.2) - 30*r(x, 0.3) +
500*r(x, 0.35) -1000*r(x, 0.37) + 1000*r(x, 0.41) - 500*r(x, 0.43) +
7.5*r(x, 0.5) - 15*r(x, 0.7) + 7.5*r(x, 0.9))}
mu=f(1:Tt/Tt)
mu=mu
beta2=5*mu/sqrt(norm(mu,'2'))
f=function(x){return(0.5 + 2*cos(4*pi*x) + 2*cos(24*pi*x))}
mu=f(1:Tt/Tt)
mu=mu-min(mu)
mu=5*mu/sqrt(norm(mu,'2'))
set.seed(12345)
N=100
Y=matrix(nrow = N,ncol = Tt)
X=matrix(rnorm(N*2),nrow=N,ncol=2)
sigma=0.3
for (i in 1:Tt) {
Y[,i]=mu[i]+X%*%rbind(beta1[i],beta2[i])+rnorm(N,0,sigma)
}
for (i in 1:N) {
Y[i,]=rpois(Tt,exp(Y[i,]))
}
r1=wavelet_fda(Y,X,0.1,trans='log')
plot(r1[1,],type='l',ylab='',main='Estimate of Beta')
lines(mu,col='grey80')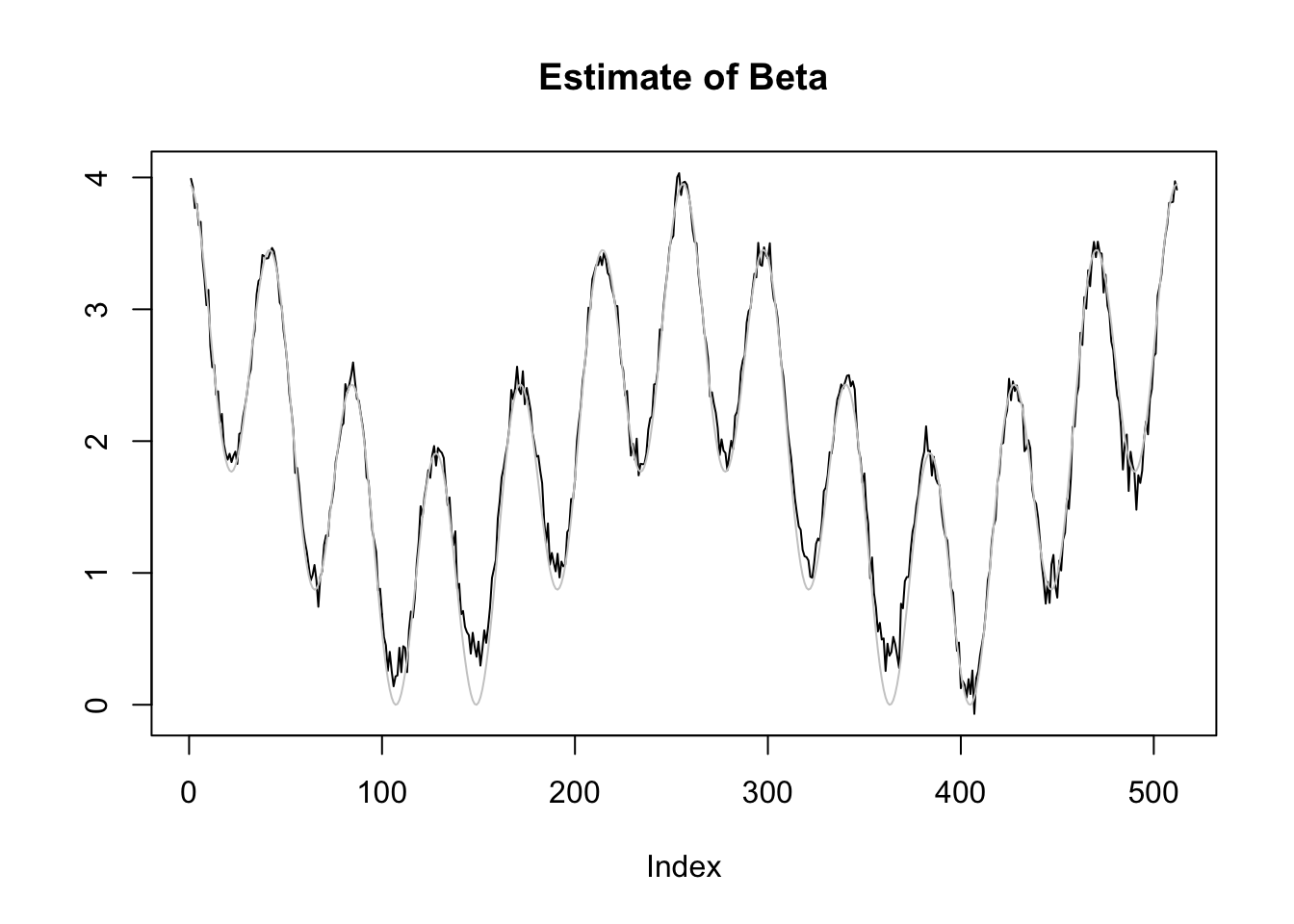
plot(r1[2,],type='l',ylab='',main='Estimate of Beta')
lines(beta1,col='grey80')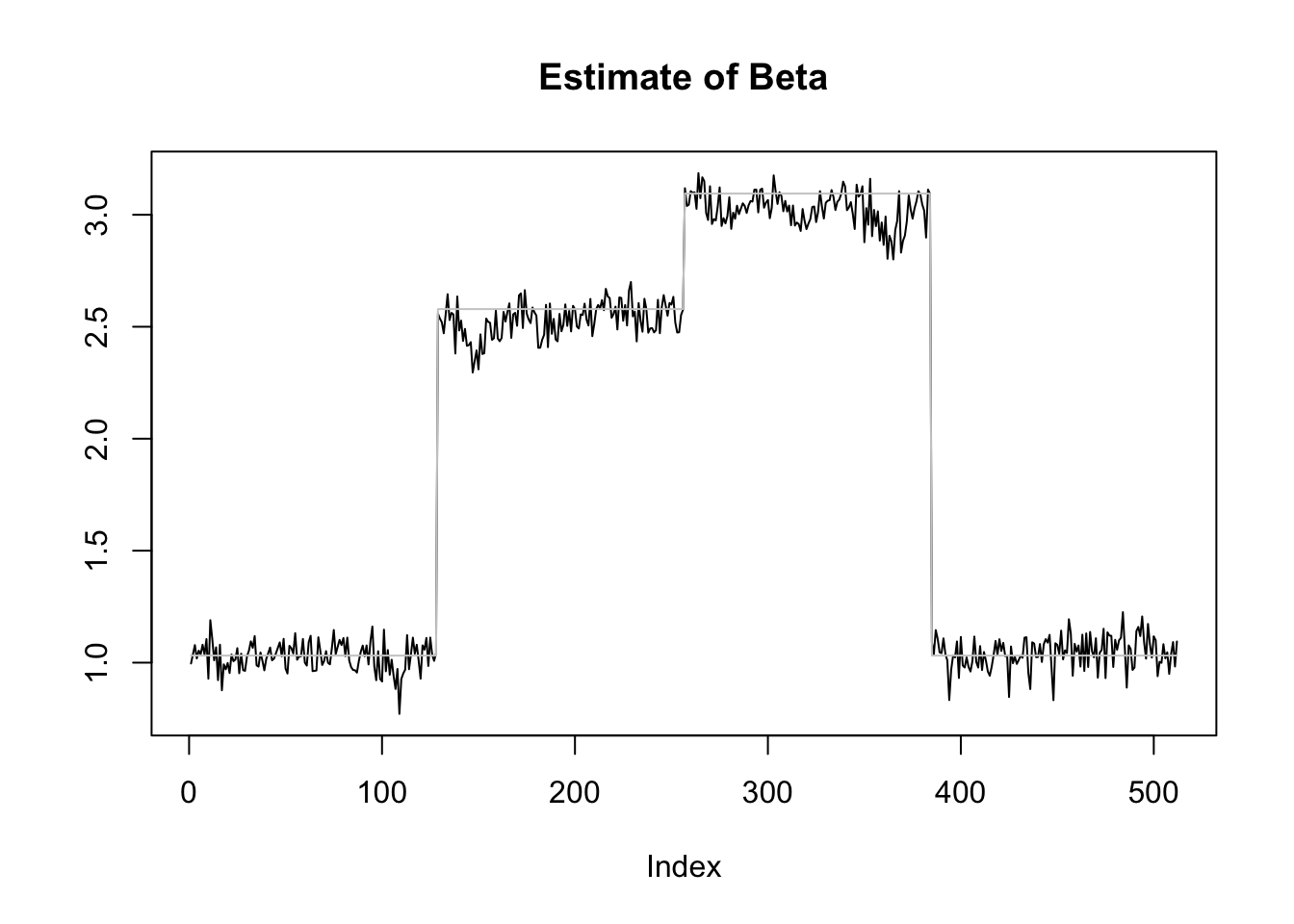
plot(r1[3,],type='l',ylab='',main='Estimate of Beta')
lines(beta2,col='grey80')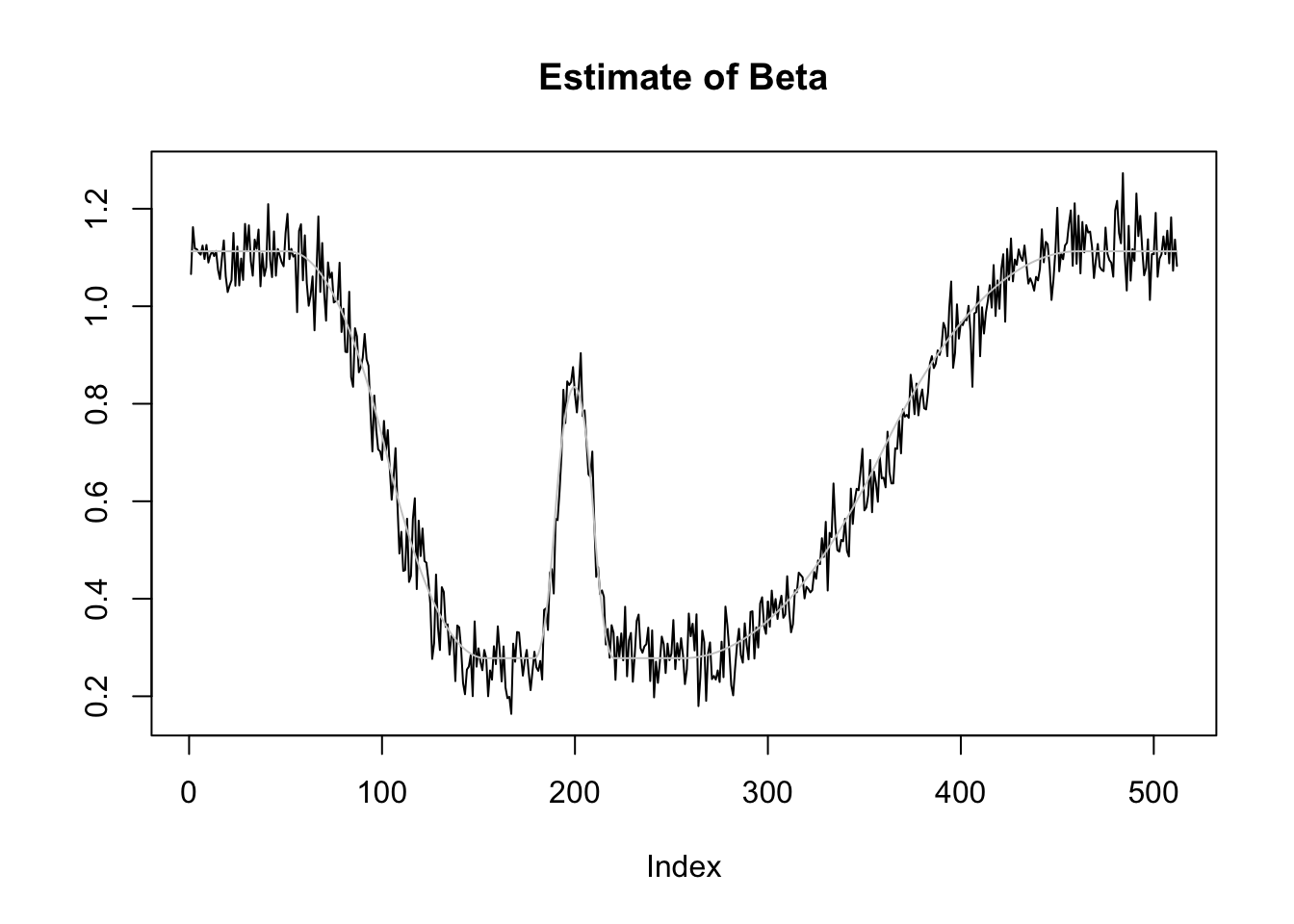
set.seed(12345)
N=100
Y=matrix(nrow = N,ncol = Tt)
X=matrix(rnorm(N*2),nrow=N,ncol=2)
sigma=0.1
for (i in 1:Tt) {
Y[,i]=mu[i]+X%*%rbind(beta1[i],beta2[i])+rnorm(N,0,sigma)
}
for (i in 1:N) {
Y[i,]=rpois(Tt,(Y[i,])^2)
}
r1=wavelet_fda(Y,X,0.1,trans='vst')
plot(r1[1,],type='l',ylab='',main='Estimate of Beta')
lines(mu,col='grey80')
plot(r1[2,],type='l',ylab='',main='Estimate of Beta')
lines(beta1,col='grey80')
plot(r1[3,],type='l',ylab='',main='Estimate of Beta')
lines(beta2,col='grey80')Session information
sessionInfo()R version 3.5.1 (2018-07-02)
Platform: x86_64-apple-darwin15.6.0 (64-bit)
Running under: macOS High Sierra 10.13.6
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/3.5/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/3.5/Resources/lib/libRlapack.dylib
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] ashr_2.2-7 wavethresh_4.6.8 MASS_7.3-51.1
loaded via a namespace (and not attached):
[1] Rcpp_1.0.0 knitr_1.20 whisker_0.3-2
[4] magrittr_1.5 workflowr_1.1.1 REBayes_1.3
[7] pscl_1.5.2 doParallel_1.0.14 SQUAREM_2017.10-1
[10] lattice_0.20-35 foreach_1.4.4 stringr_1.3.1
[13] tools_3.5.1 parallel_3.5.1 grid_3.5.1
[16] R.oo_1.22.0 git2r_0.23.0 iterators_1.0.10
[19] htmltools_0.3.6 assertthat_0.2.0 yaml_2.2.0
[22] rprojroot_1.3-2 digest_0.6.18 Matrix_1.2-14
[25] codetools_0.2-15 R.utils_2.7.0 evaluate_0.11
[28] rmarkdown_1.10 stringi_1.2.4 compiler_3.5.1
[31] Rmosek_8.0.69 backports_1.1.2 R.methodsS3_1.7.1
[34] truncnorm_1.0-8 This reproducible R Markdown analysis was created with workflowr 1.1.1